
The Structure of the Atom GCSE Physics Science) AQA
Most of the mass of an atom is in the nucleus, while the orbiting electrons account for an atom's size. As a result, an atom consists largely of empty space. Rutherford called his description the "planetary model" of the atom. Figure 2.3.2 2.3. 2 shows how this model explains the experimental results.

Atomic Structure Broad Learnings
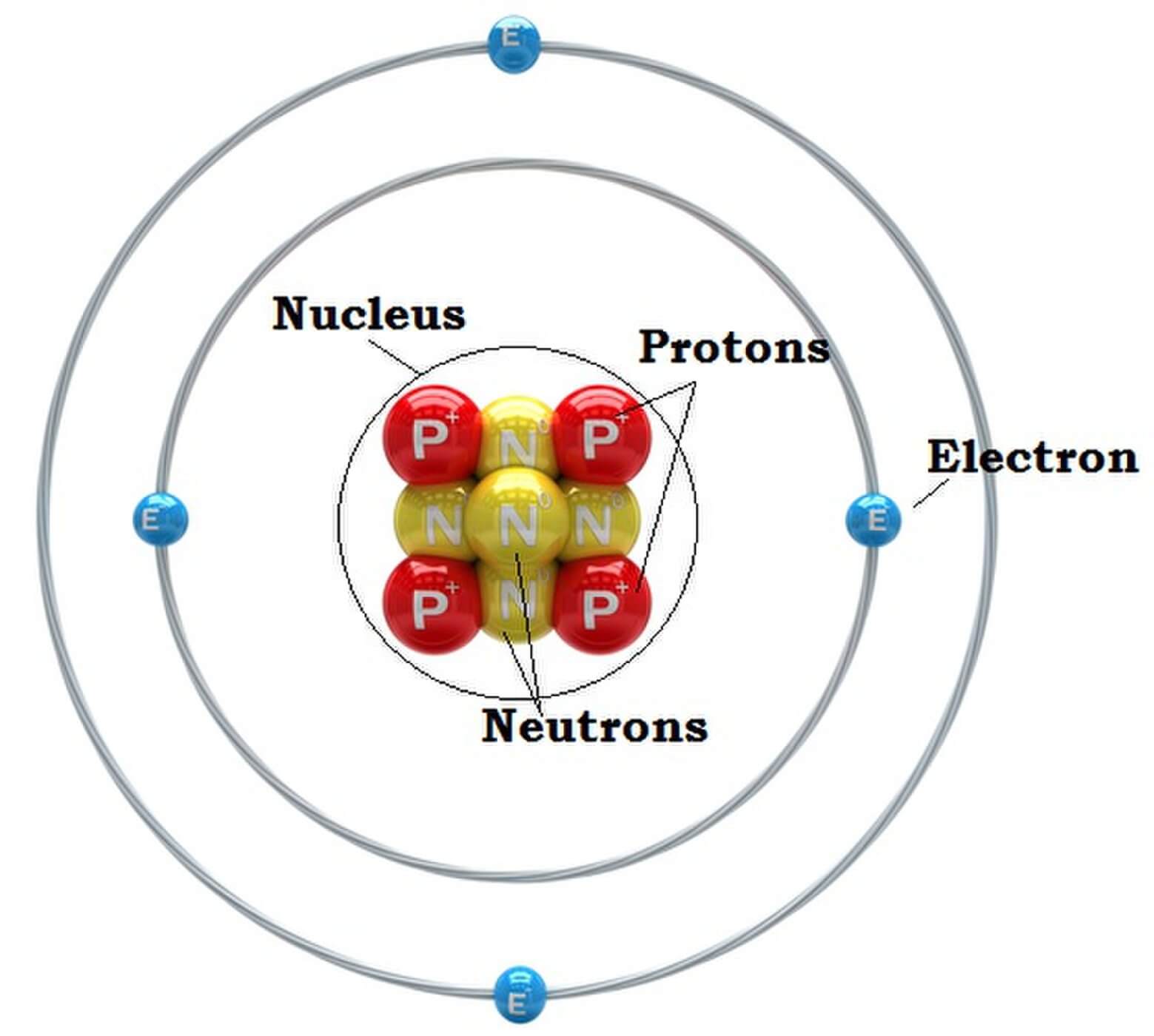
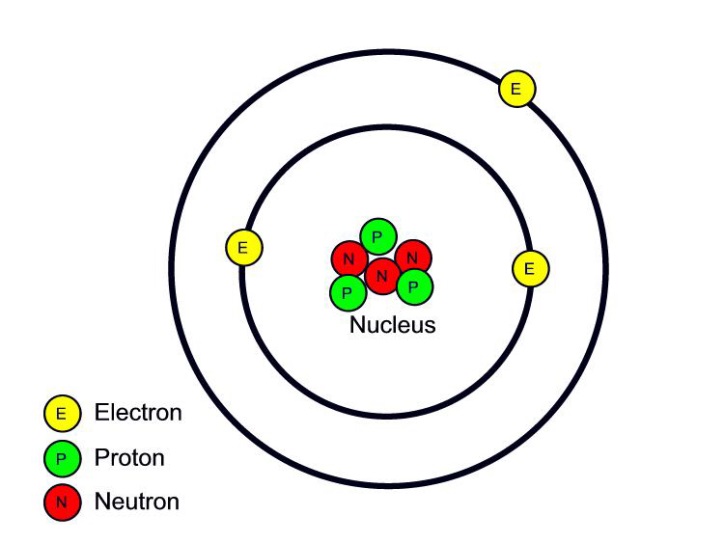
Figure 2.2.1 2.2. 1: The Structure of the Atom. Atoms have protons and neutrons in the center, making the nucleus, while the electrons orbit the nucleus. The modern atomic theory states that atoms of one element are the same, while atoms of different elements are different.

Chemical bonding Atomic structure and bonding Britannica
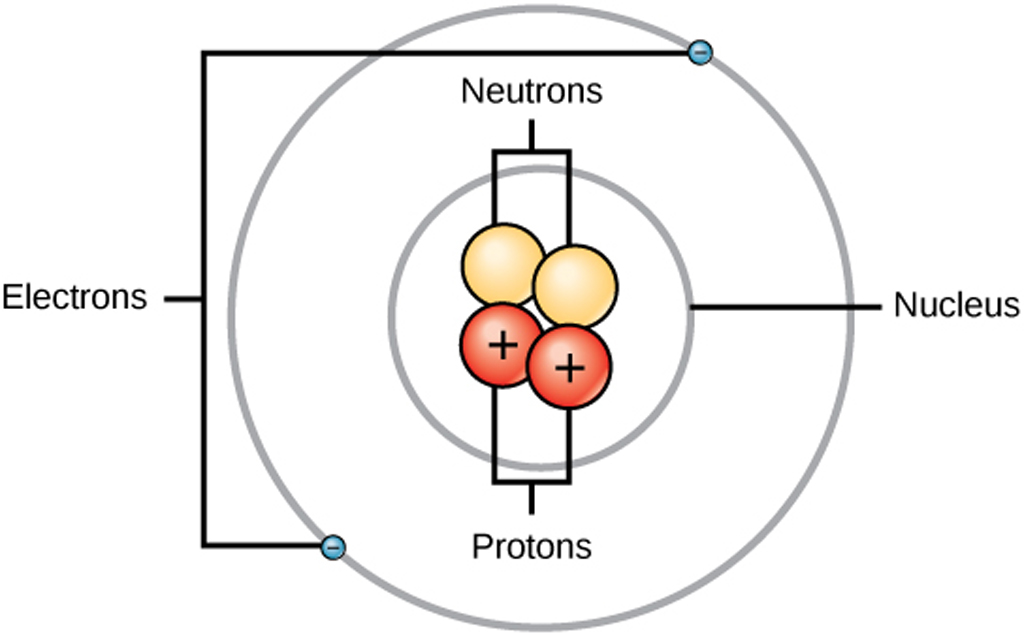
Atom The atom is the basic particle of the chemical elements. An atom consists of a nucleus of protons and generally neutrons, surrounded by an electromagnetically-bound swarm of electrons. The chemical elements are distinguished from each other by the number of protons that are in their atoms.

Structure Of An Atom Class 9 Science Notes Leverage Edu
Atoms are the foundation of chemistry. They are the basis for everything in the Universe. As you know, matter is composed of atoms. Solids are made of densely packed atoms while gases have atoms that are spread out. We're going to cover basics like atomic structure and bonding between atoms. As you learn more, you can move to the reactions and.
What is an Atom? Definitions & Examples Let us learn Basics News Bugz
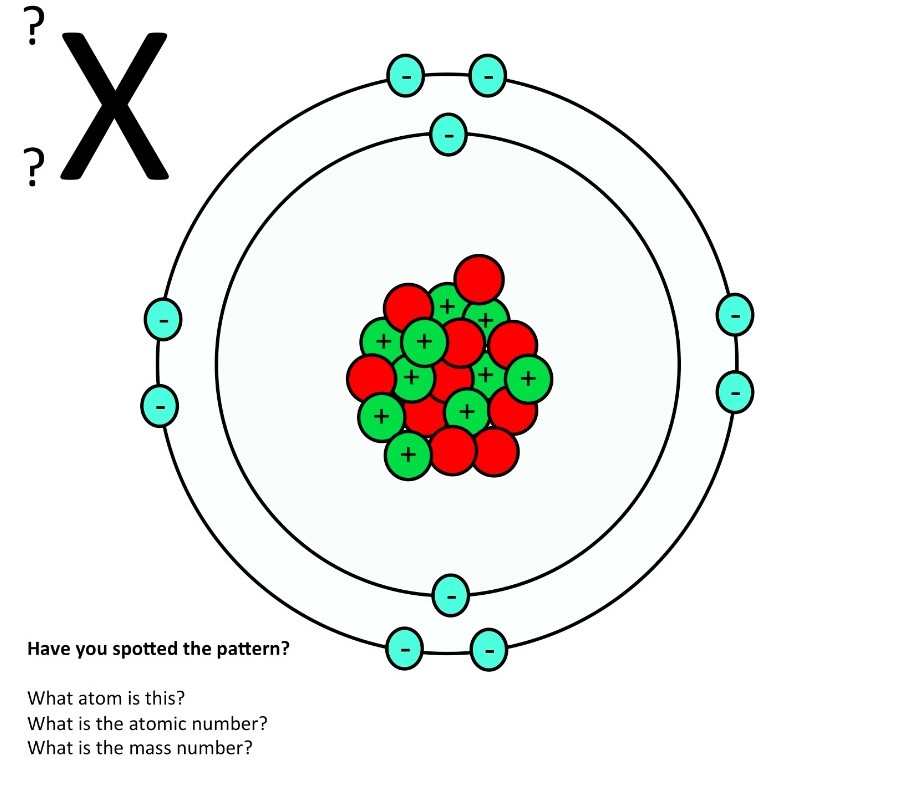
Here are electron shell atom diagrams for the elements, ordered by increasing atomic number . For each electron shell atom diagram, the element symbol is listed in the nucleus. The electron shells are shown, moving outward from the nucleus.
Atoms and Atomic Structure HubPages
The atomic structure of these building blocks is very interesting. The protons and neutrons are located in the center of the atom, while the electrons are quite far from the center. The number of protons in the nucleus of an atom determines its atomic number and its identity as a specific element. For example, all hydrogen atoms have one proton.
/GettyImages-141483984-56a133b65f9b58b7d0bcfdb1.jpg)
Basic Model of the Atom Atomic Theory
nucleus . This is surrounded by electrons arranged in shells. The nucleus is tiny compared to the atom as a whole: the radius of an atom is about 0.1 nm (1 × 10 -10 m) the radius of a nucleus (1 ×.
Structure of an Atom Structure & Use of Electron & Proton in Electronics
Atom. Atoms are tiny particles that form the basic building blocks of all matter in the universe, whether solid, liquid, or gas. All living organisms and nonliving objects found on Earth are made of trillions and trillions of atoms. The smaller particles that make up an atom are known as subatomic particles. The term 'atom' was derived from.
Atomic structure teaching resources the science teacher
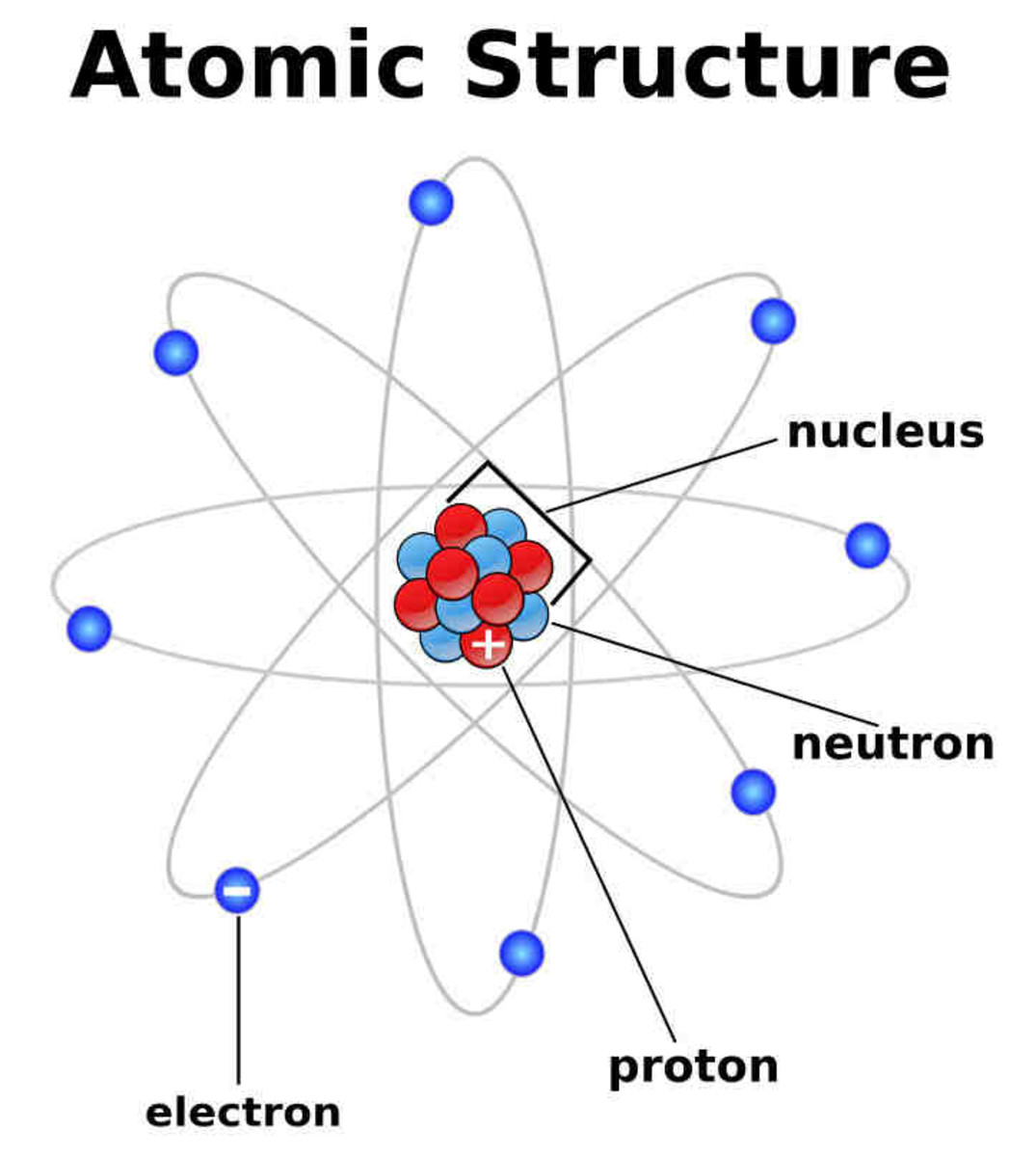
Basic Diagram of an Atom Most of an atom is just empty space and consists of a positively charged nucleus of protons and neutrons surrounded by a cloud of negatively charged electrons. The center of an atom is the nucleus and one or more electrons surrounding the nucleus.
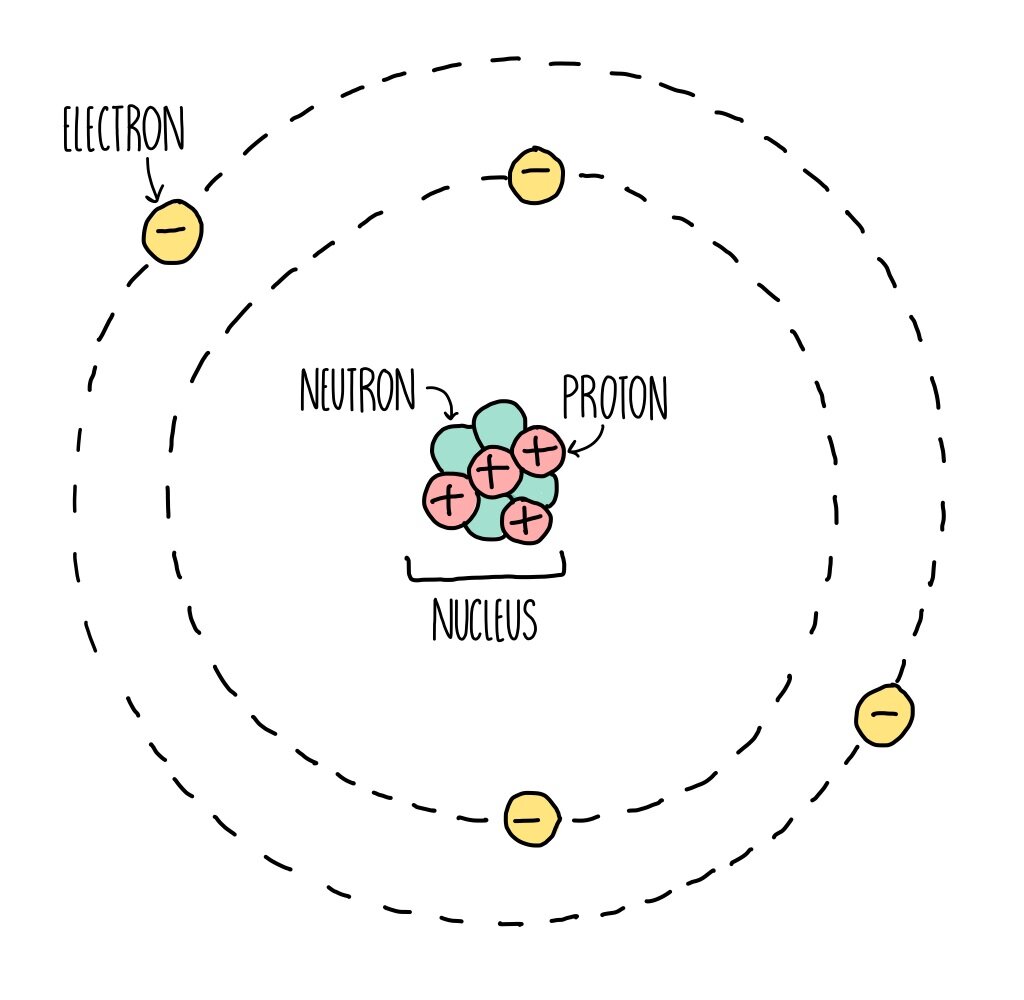
Atomic Structure (GCSE) — the science hive
Physical Chemistry (Essentials) - Class 11 8 units · 52 skills. Unit 1 Welcome to physical chemistry. Unit 2 Structure of atom. Unit 3 Some basic Concepts of Chemistry. Unit 4 Redox reactions. Unit 5 Gaseous state. Unit 6 Thermodynamics. Unit 7 Chemical Equilibrium. Unit 8 Ionic equilibrium.

Atomic Structure & The Changing Models of Atom
The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2. The helium atom contains two protons and two electrons. The first electron has the same four quantum numbers as the hydrogen atom electron ( n = 1, l = 0, ml = 0, ms = +12 m s = + 1 2 ).
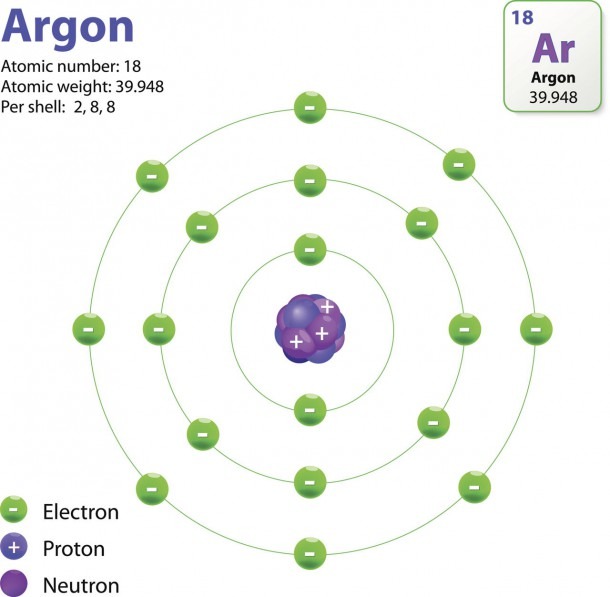
The Structure Of An Atom Explained With A Labeled Diagram Best
Figure 2.4. 1: The Geiger-Marsden Experimental Setup. Experiments using this setup were used to investigate the structure of atoms. In this experiment, most of the particles traveled straight through the foil, but some alpha particles were deflected off to one side. Some were even deflected back toward the source.
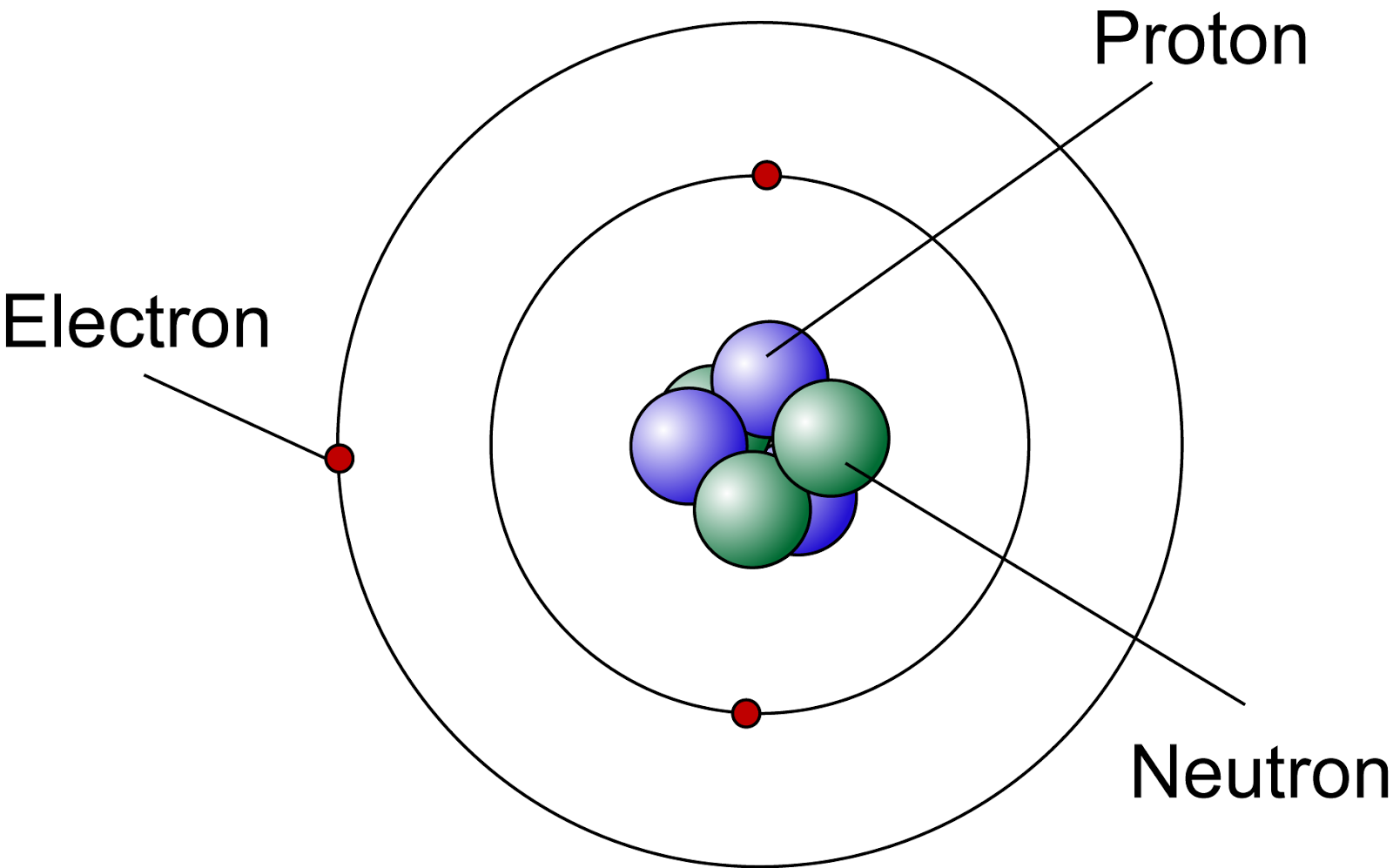
Lets Get Inside An Atom!! The Science Station
The atomic structure refers to the structure of an atom comprising a nucleus (centre) in which the protons (positively charged) and neutrons (neutral) are present. The negatively charged particles called electrons revolve around the centre of the nucleus. Download Complete Chapter Notes of Structure of Atom Download Now
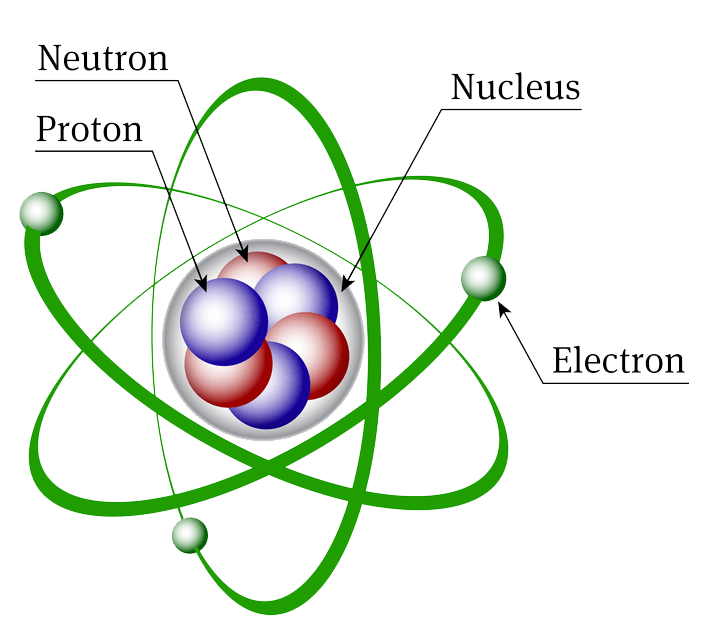
Atomic Structure Biochemistry
Atomic structure is the structure of an atom that consists of a nucleus (the centre), protons (positively charged), and neutrons (neutral). The electrons are negatively charged particles that orbit the nucleus's centre. Democritus came up with the concept that matter is composed of atoms.

Learn the Parts of an Atom
All atoms are roughly the same size, whether they have 3 or 90 electrons. Approximately 50 million atoms of solid matter lined up in a row would measure 1 cm (0.4 inch). A convenient unit of length for measuring atomic sizes is the angstrom (Å), defined as 10 −10 metre. The radius of an atom measures 1-2 Å.
Atomic structure WGHS Junior Science
Map: Chemistry - The Central Science (Brown et al.) 2: Atoms, Molecules, and Ions